Implantable neurostimulators american national standard i o is is a preview edition of an aami guidance document and is intended to allow potential purcasers to evaluate te content of te document before maing a purcasing decision.
Iso standards for medical devices pdf.
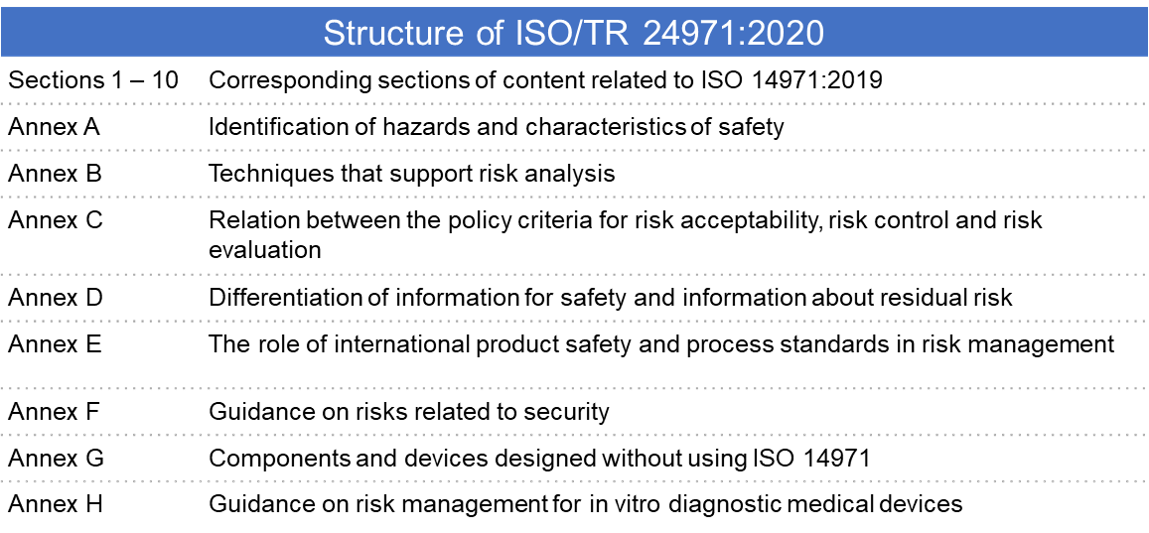
This document specifies terminology principles and a process for risk management of medical devices including software as a medical device and in vitro diagnostic medical devices the process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the medical device to estimate and evaluate the associated risks to control these.
This article explains the method starting with standards from the international organization for standardization iso adopted and recognized in various regulatory systems.
Iso 13485 2016 medical devices quality management systems requirements for regulatory purposes.
Iso 13485 is designed to be used by organizations involved in the design production installation and servicing of medical devices and related services.
Iso 14155 was developed by working group wg 4 clinical investigations of medical devices in humans of iso technical committee iso tc 194 biological and clinical evaluation of medical devices the secretariat of which is held by din iso s member for germany.
These standards are very relevant for medical devices and encompass virtually every aspect of device design and implementation from device inspection requirements to guidelines for medical device labels.
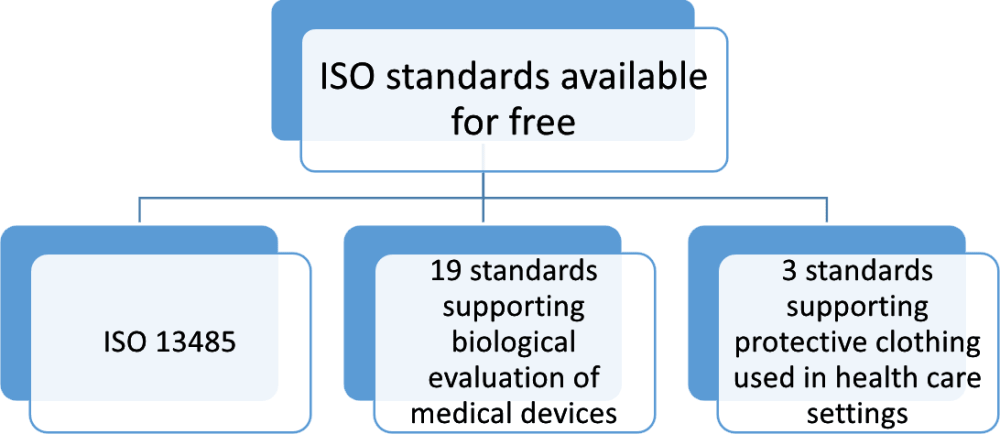
The standards are available in read only format and you can find the links here.
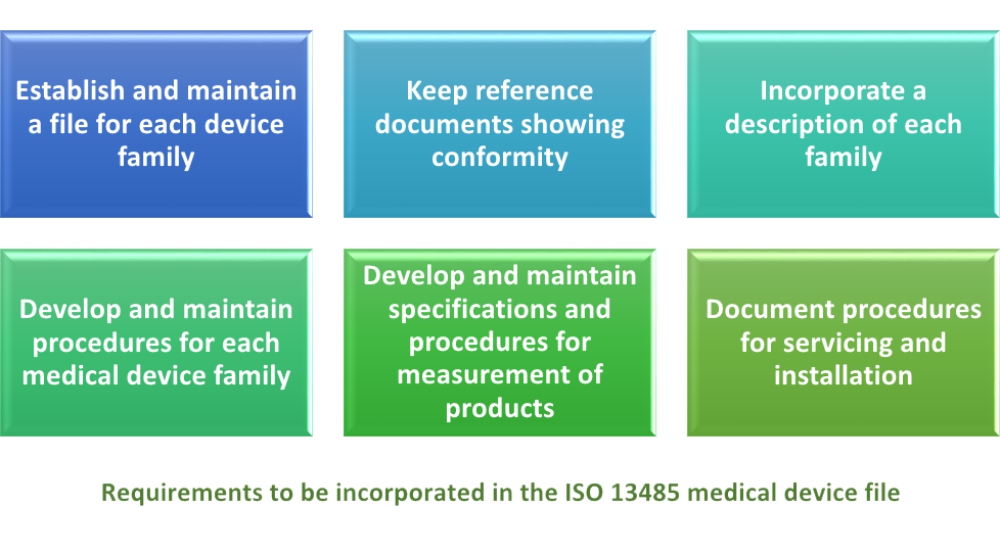
It is designed to be used by organizations throughout the life cycle of a medical device from initial concep tion to production and post production including final decommission and disposal.
The article uses iso 13485 2003 and iso 14971 2007 as illustrations p.
Terminology and performance requirements for micro organisms risk.
Iso 374 5 2016 protective gloves against dangerous chemicals and micro organisms part 5.
2017 implants for surgery active implantable medical devices part 3.
Iso 13485 2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements.
O for example iso 13485 establishes the requirements for a quality management system.
It is available for purchase from your national iso member or through the iso store.
Iso 13485 medical devices quality management systems requirements for regulatory purposes is an internationally agreed standard that sets out the requirements for a quality management system specific to the medical devices industry.
Medical devices application of usability engineering to medical devices.